The flow of energy in ecosystems is one-way. In contrast, the nutrients which are needed to produce organic material are circulated round the system and are re-used several times.
- All natural elements are capable of being absorbed by plants, usually as gases from the air or as soluble salts from the soil, but only oxygen, carbon, hydrogen and nitrogen are needed in large quantities. These substances are known as macronutrients and form the basis of fats, carbohydrates and proteins.
- Other nutrients, such as magnesium, sulphur and phosphorus are needed in minute amounts and are known as micronutrients.
- Nutrient cycles can be presented in the framework of a model in which each cycle has a reservoir pool, which is a large, slow-moving non-biological component, and an exchange pool, which is a smaller, more active portion where the nutrient is exchanged between biotic and abiotic parts of the ecosystem. There are two basic types of cycle, gaseous ones, in which the reservoir pool is the atmosphere, and sedimentary ones, in which the reservoir pool is the Earth’s crust.
- Another name of nutrient cycling is biogeochemical cycles (bio: living organism, geo: rocks, air, and water).
Water Cycle
- Water undergoes a cycle from the ocean to land and land to
- The hydrological cycle describes the movement of water on, in, and above the
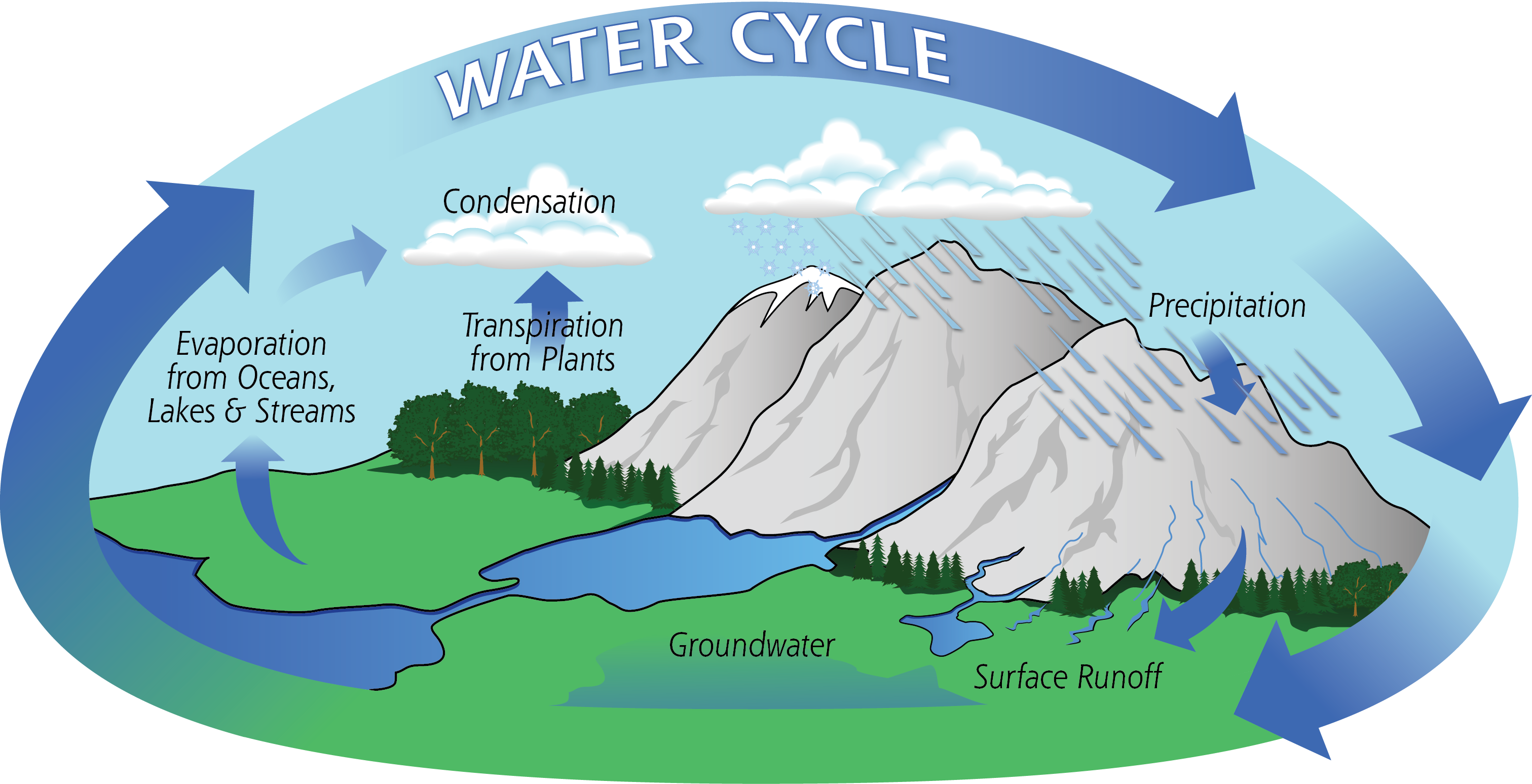
- The distribution of water on earth is quite uneven. Many locations have plenty of water while others have very limited quantity.
- The hydrological cycle is the circulation of water within the earth’s hydrosphere in different forms e. the liquid, solid and the gaseous phases.
About 71 per cent of the planetary water is found in the oceans. The remaining is held as freshwater in glaciers and icecaps,
groundwater sources, lakes, soil moisture, atmosphere, streams and within life. Nearly 59 per cent of the water that falls on land returns to the atmosphere through evaporation from over the oceans as well as from other places. The remainder runs-off on the surface, infiltrates into the ground or a part of it becomes glacier.
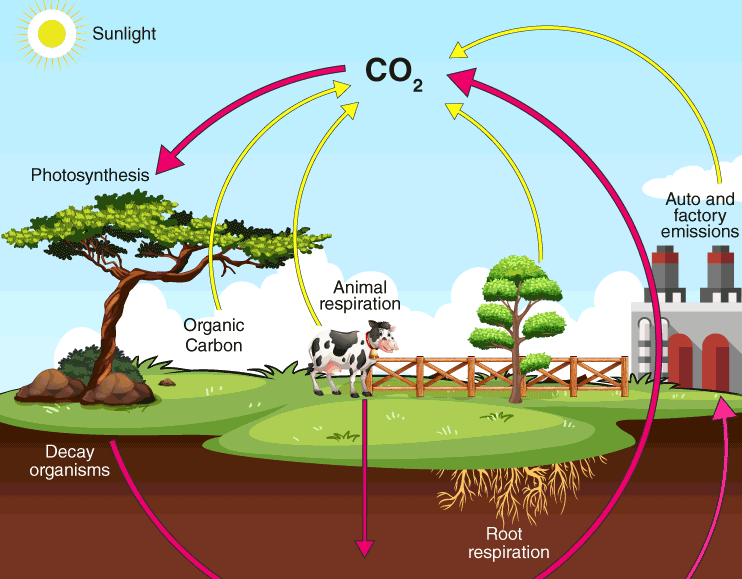
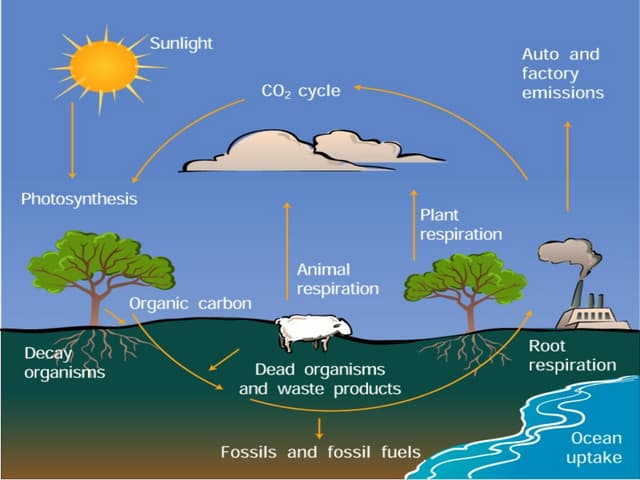
Carbon Cycle
- Carbon cycle is mainly the conversion of carbon

- This conversion is initiated by the fixation of carbon dioxide from the atmosphere through photosynthesis.
- Such conversion results in the production of carbohydrate, glucose that may be converted to other organic compounds.
Figure : Cycle
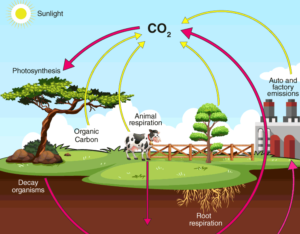 |
- Some of the carbohydrates are utilised directly by the plants
- During the process, more Carbon dioxide is generated and is released through its leaves or roots during the
- The remaining carbohydrates not being utilised by the plant become part of the plant
- Plant tissues are either being eaten by the herbivorous animals or get decomposed by the
- The herbivores convert some of the consumed carbohydrates into carbon dioxide for release into the air through respiration.
- The micro-organisms decompose the remaining carbohydrates after the animal dies.
- The carbohydrates that are decomposed by the micro- organisms then get oxidised into carbon dioxide and are returned to the atmosphere.
The Phosphorus Cycle
- The phosphorus cycle is an example of a sedimentary cycle which is easily disrupted.
- Phosphates in the soil are taken into plants for protein synthesis and are passed through the food chains of
- When plant and animal bodies and their excretory products decompose, the phosphorus is released to the soil where it can either be taken back into plants or washed out by rainfall into drainage systems which ultimately take it to the sea.
- If this happens it will be incorporated in marine sediments and so lost from the exchange

- One important route for the rapid return of phosphorus from these sediments occurs where there are upwelling ocean currents. These bring phosphorus to the surface waters, where it is taken into marine food
- The depletion of phosphorus from the exchange pool is compensated very slowly by the release of the element from the phosphate rocks of the reservoir pool. This occurs by the process of erosion and
- The phosphorus cycle can be easily disrupted by the use of phosphate fertilisers in modern
- Most manufactured phosphate fertilisers are produced from phosphate rocks but are rapidly lost from the exchange pool to marine deposits as they are easily leached from the soil.
The Nitrogen Cycle
- The nitrogen cycle is an example of a gaseous
- It is probably the most complete of the nutrient cycles.
- The reservoir pool is the atmosphere and the exchange pool operates between organisms and the soil.
- Atmospheric nitrogen in the reservoir pool cannot be used directly by most plants. It has to be made into a chemical compound such as a nitrate before it is available to the exchange
- Nitrates in the soil are absorbed by plants and pass through food chains.
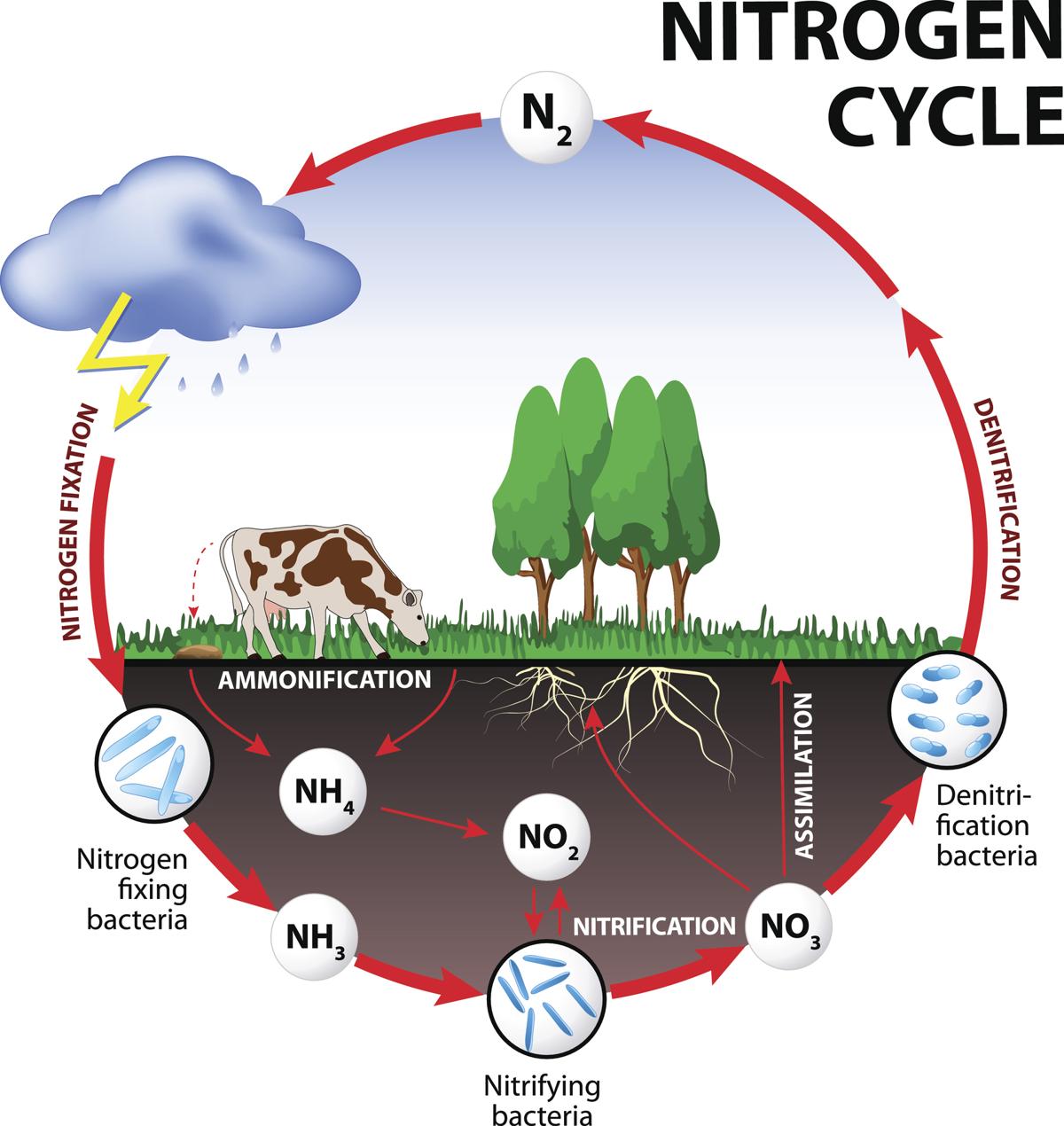
- Ultimately they are released as ammonia when organic material is decomposed.
- The ammonia is changed back to nitrates by the action of bacteria.
- If the nitrates are not reabsorbed by plants they may be lost from the exchange pool in two ways: first, by leaching from the soil to shallow marine sediments (in this case they may be returned in the droppings of marine birds in the same way as phosphorus); second, nitrates may be lost from the soil by being broken down by denitrifying bacteria, and the nitrogen contained in them being released to the atmosphere.
- The conversion of gaseous nitrogen to nitrate occurs in two main ways. Some can be fixed by electrical action during thunderstorms, but most is converted by nitrogen-fixing organisms. These are mostly bacteria, algae and fungi, and either operates by themselves in the soil or in an association with a plant, particularly those in the legume family, such as clover.
The Oxygen Cycle
The cycling of oxygen is a highly complex process. Oxygen occurs in a number of chemical forms and combinations.
It combines with nitrogen to form nitrates and with many other minerals and elements to form various oxides such as the iron oxide, aluminium oxide and others.
Much of oxygen is produced from the decomposition of water molecules by sunlight during photosynthesis and is released in the atmosphere through transpiration and respiration processes of plants.
Other Mineral Cycles
Other than carbon, oxygen, nitrogen and hydrogen being the principal geochemical components of the biosphere, many other minerals also occur as critical nutrients for plant and animal life. These mineral elements required by living organisms are obtained initially from inorganic sources such as phosphorus, sulphur, calcium and potassium.
They usually occur as salts dissolved in soil water or lakes, streams and seas. Mineral salts come directly from the earth’s crust by weathering where the soluble salts enter the water cycle, eventually reaching the sea.
Other salts are returned to the earth’s surface through sedimentation, and after weathering, they again enter the cycle. All living organisms fulfil their mineral requirements from mineral solutions in their environments. Other animals receive their mineral needs from the plants and animals they consume. After the death of living organisms, the minerals are returned to the soil and water through decomposition and flow.



